If system accuracy, efficiency, and reliability are critical, designing sensor nodes for wireless data transmission for remote monitoring can be a considerable challenge.
The pH of the solution is a measure that many industries need to consider. The purpose of the reference design we shared today is to evaluate the characteristics of the pH glass probe to solve the different challenges of hardware and software design, and to propose a radio frequency transceiver. The module wirelessly transmits data from the probe solution.
Part 1: pH probe
pH definition
The aqueous solution can be classified into three types: acidic, basic and neutral. In chemistry, pH is measured by a numerical scale called pH. According to the definition of the Carlsberg Foundation, the pH represents the hydrogen ion concentration. This scale is a logarithmic scale ranging from 1 to 14. The mathematical expression for pH is:
pH = –log(H+)
Therefore, if the hydrogen ion concentration is 1.0 × 10–2 mol/L, then
pH = –log(1.0 × 10–2) = 2
The pH of an aqueous solution such as distilled water is 7, which is a neutral value. A solution having a pH of less than 7 is an acidic solution, and a solution having a pH greater than 7 is an alkaline solution. The logarithmic scale reflects the acidity of one solution relative to another.
For example, a solution having a pH of 5 has 10 times the acidity of a solution having a pH of 6 and 1000 times that of a solution having a pH of 8.
pH indicator
There are many ways to measure the pH of an aqueous solution, including through a litmus paper indicator or using a glass probe.
Litmus paper
The litmus paper indicator is usually made of dye liquor extracted from the lichens and can be used to indicate pH levels. Upon contact with the solution, the test paper undergoes a chemical reaction that causes its color to change, thereby indicating the pH level. This category generally includes two methods:
Comparing the standard color corresponding to the known pH value with the color of the indicator immersed in the test liquid with the buffer solution;
The pH test paper is first immersed in the indicator and then immersed in the test liquid to compare its color to the standard color. Although the above two methods are easy to implement, the temperature and impurities in the test solution are liable to cause errors.
pH glass probe
The most commonly used pH indicator is the pH probe. It consists of a glass measuring electrode and a reference electrode. A typical glass probe consists of a glass film and a solution of hydrochloric acid (HCl) enclosed therein. Inside the housing is an AgCl-plated silver wire that acts as a reference electrode and is in contact with the HCL solution. Hydrogen ions outside the glass film diffuse through the glass membrane, replacing the corresponding amount of sodium ions (Na+), and sodium ions are generally present in most glasses. This positive ion is very sensitive and is mostly limited to the film on the lower side of the glass surface. The excess charge of Na+ produces a voltage at the output of the sensor.
The probe is similar to a battery. When the probe is placed in solution, the measuring electrode produces a voltage whose magnitude depends on the activity of the hydrogen in the solution and then compares this voltage to the potential of the reference electrode. As the acidity of the solution increases (the pH becomes lower), the glass electrode potential is positively enhanced relative to the reference electrode (+mV); as the solution becomes alkaline (the pH becomes higher), the glass electrode potential is relative to the reference electrode. Negative enhancement (-mV). The difference between these two electrodes is the measured potential. Ideally, a typical pH probe will produce 59.154 mV/pH units at 25 ° C, usually expressed in the Nernst equation as follows:
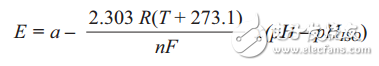
among them:
E = hydrogen electrode voltage, activity unknown
a = ±30 mV, zero tolerance
T = ambient temperature (25 ° C) n = 1 (25 ° C), the price (number of charges on the ion)
F = 96485 Coulombs/mole, Faraday constant
R = 8.314 volts-coulomb/°K mole, Avogadro's number
pH = hydrogen ion concentration of unknown solution
pHISO = 7, reference hydrogen ion concentration
The equation shows that the voltage produced depends on the acidity and alkalinity of the solution and varies with hydrogen ion activity in a known manner. A change in the temperature of the solution changes the activity of its hydrogen ions. When the solution is heated, the hydrogen ion moves faster, resulting in an increase in the potential difference between the two electrodes. In addition, when the solution is cooled, the hydrogen activity is lowered, resulting in a decrease in the potential difference. Ideally, the electrode will produce zero volts when placed in a pH 7 buffer solution. The specifications for a typical pH probe are shown in the table below.
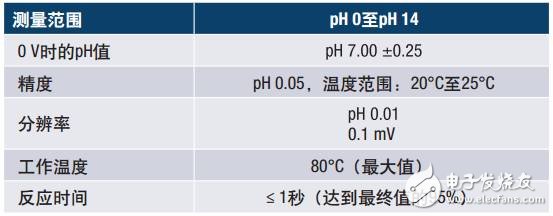
Table 1. Typical Specifications for pH Glass Probes
pH probes play an important role in this study because data reliability depends on the accuracy and reliability of the sensor. There are two important factors to consider when choosing a pH probe:
Stabilization time after buffer solution temperature change
Stabilization time after pH change
As an example, the following data is taken from Jenway's application note "Jenway High Performance pH Electrode Evaluation", which shows the stability of the probe after a temperature change under given test conditions. A solution was prepared which had a pH of 7 at 20 ° C and a pH of 4 at 60 ° C. The electrodes were allowed to stabilize in pH 7 buffer stirred at 200 rpm. The electrode was then rinsed with deionized water and transferred to an aliquot of pH 4 buffer for 4 minutes. The electrode was again washed with deionized water and then returned to pH 7 buffer. Evaluate the time it takes for the reading to remain stable for 10 seconds. Repeat the test three times for each probe.
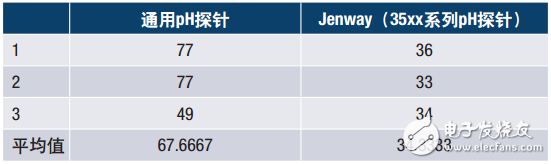
Table 2. Settling time after buffer solution temperature change
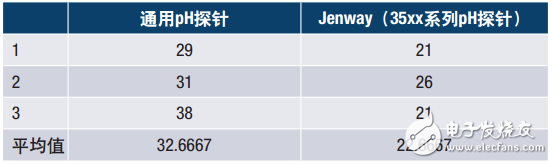
Table 3. Settling time after pH change of buffer solution
The performance of the Jenway probe is up to 50% faster than the universal pH probe given the conditions given. It is advantageous to use a meter like this because the sample throughput rate is high and the time required to analyze the data is greatly reduced.
Sensor analog signal conditioning circuit
In order to understand the signal conditioning circuit, the equivalent circuit diagram of the sensor probe must be known. As described in the previous section, the pH probe is made of glass and can form extremely high resistances ranging from 1 MΩ to 1 GΩ, acting as a resistor in series with the pH voltage source, as shown in Figure 1.
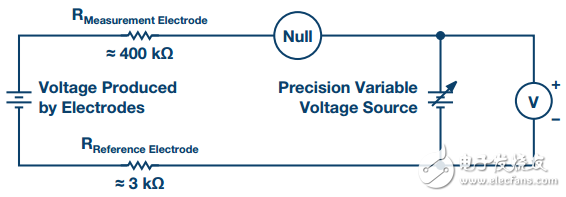
Figure 1. pH probe equivalent circuit configuration
Even with very small circuit currents flowing through the high resistance of the devices in the circuit (especially the glass film of the measuring electrode), these resistors produce a relatively large voltage drop, which severely reduces the voltage measured by the meter. To make matters worse, the voltage difference produced by the measuring electrode is very small, in the millivolt range (ideally, room temperature corresponds to 59.16 mV per pH unit). The meters used for this task must be very sensitive and have very high input resistance.
Analog to digital conversion
For such applications, when a given sensor response time, the data sampling rate would be a problem. Assuming a sensor resolution of 0.001 V rms and an ADC full-scale voltage range of 1 V, an effective resolution of 9.96 bits is achieved without the need for a high resolution ADC. The noise-free resolution unit is a bit and is defined by:
Noise-free resolution = log2 [full-scale input power range / sensor peak-to-peak voltage output noise]
The ADC sampling rate can be an important factor for low power applications because the sampling rate of the ADC is directly related to power consumption. The typical ADC sampling rate can be set to its lowest throughput rate when the sensor's response time is constant. A microcontroller with an integrated ADC can be used to reduce the number of components.
Part II: TransceiverTransceivers are required to transfer pH and temperature data, and microcontrollers are required to control transceivers. The choice of transceivers and microcontrollers involves some important considerations. The following factors must be considered when selecting a transceiver:
working frequency

Designing the RF transmission must determine the operating frequency (OF), whether the sub-GHz or 2.4 GHz frequency can meet the application requirements. In applications that require high data rates and wide bandwidths such as Bluetooth, the 2.4 GHz frequency is the best choice. However, industrial applications typically use sub-GHz frequencies because the proprietary protocols available can easily provide a network link layer. Proprietary systems primarily use ISM frequencies in the sub-GHz range, namely 433 MHz, 868 MHz and 915 MHz.
The maximum distance range Sub-1 GHz frequency supports long distance and high power transmission of more than 25 km. When used in a point-to-point or star topology, these frequencies effectively penetrate walls and other obstacles.
Data rate
The data rate also needs to be determined, which affects the transceiver's transmission distance capability and power consumption. When the data rate is high, the power consumption is low, and it can be used for short-distance transmission. When the data rate is low, the power consumption is high, and it can be used for long-distance transmission. To reduce power consumption, it is a good idea to increase the data rate because it consumes current in bursts in a short period of time, but it also reduces the radio coverage distance.
Transceiver power consumption
Transceiver power consumption is very important for battery-powered applications. This is also a consideration in many wireless applications because it determines the data rate and range of distances. The transceiver has two power amplifier (PA) options to provide greater flexibility in use. A single-ended PA can output up to 13 dBm of RF power, and a differential PA can output up to 10 dBm of power.
Table 4 summarizes the relationship between some PA output power and transceiver IDD current consumption. For the sake of completeness, the current consumption of the receive mode is also given in the table.
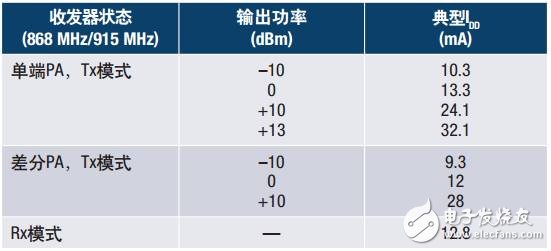
Table 4. Summary of PA Output Power and Transceiver IDD Current Consumption
license
Sub-GHz includes the license-free ISM bands of 433 MHz, 868 MHz and 915 MHz. It is widely used in industry and is ideal for a wide range of wireless applications. It can be used in different parts of the world because it complies with European ETSI EN300-220 regulations, North American FCC Part 15 regulations and other similar regulatory standards.
Part III: Microcontroller
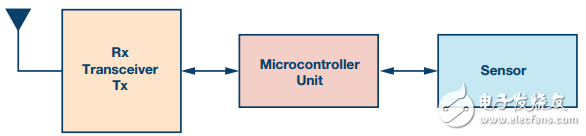
Figure 2. Block diagram of wireless sensor data acquisition and transmission
The choice of microcontroller must consider the following factors:
Peripheral
The microcontroller should integrate peripherals such as the SPI bus. Both the transceiver and the pH reference design board are connected via SPI, so two SPI peripherals are required.
Memory
The microcontroller performs protocol processing and sensor interface tasks with the appropriate size of memory. Flash and RAM are two very important components of a microcontroller. To ensure that the system does not run out of storage, use 128 kB of memory. This will definitely allow application and software algorithms to run smoothly and leave room for possible upgrades and feature additions (to eliminate system problems).
Architecture and processing power
The microprocessor must be fast enough to handle complex calculations and processes. The system uses a 32-bit microprocessor. Although a lower number of processors may be possible, the system chose to use 32 bits to support potentially higher application and algorithm requirements.
Power consumption
The power consumption of the microprocessor should be very low. For applications that rely on battery power and must run for years without maintenance, power consumption is critical.
Part IV: Other System ConsiderationsError check

The communications processor appends the CRC to the payload in transmit mode and the CRC in receive mode. The payload data plus the 16-bit CRC can be encoded/decoded using Manchester encoding techniques.
cost
The system should use the fewest components and the smallest board size, as these are often the deciding factors when cost is one of the key requirements. Do not use discrete devices, you must consider an integrated solution consisting of MCUs and wireless devices. This eliminates the design challenges of interconnecting the radio and MCU, simplifies board design, makes the design process more straightforward, and shortens the bond wire, making it less susceptible to interference. With a single chip that combines ARM® Cortex®-M MCUs and radio transceivers, you can reduce the number of board components, simplify board layout, and lower total cost.
calibration
Performing a calibration routine is one of the key aspects of achieving high precision. One feature of the pH solution described by the Nernst equation is that it is highly temperature dependent. The sensor probe only gives a constant offset, which can be considered constant at all temperature levels. Because of its high temperature dependence, the system must have a sensor that determines the temperature of the solution.
Methods such as direct substitution into the Nernst equation can be used, but due to the non-ideal nature of the solution, some degree of error may occur. This method only needs to measure the system's offset and the temperature of the unknown solution. To determine the offset introduced by the sensor, a buffer solution with a pH of 7 is required. Ideally, the sensor should produce a 0 V output. The ADC reading will be the system offset voltage. Typical pH probe sensors can be offset up to ±30 mV.
Another method is often used in practice, using a plurality of buffer solutions to set points to construct a general linear or nonlinear equation. In this routine, two additional NIST-certified and traceable pH buffer solutions are required. The pH of these two additional buffer solutions should differ at least.
The method of performing calibration by buffer solution is as follows:
? Step 1: pH probe electrode assembly after removal from a first buffer solution and deionized water or distilled water, with the temperature sensor is immersed in the second buffer solution chosen.
? Step 2: Step 2 was repeated, except that the third buffer solution.
? Step 3: According to equation established using the selected value of the buffer solution was measured.
A plurality of mathematical equations can be used to derive the calibration equation. One of the commonly used formulas is the point-oblique linear equation. This equation uses two points obtained during calibration:
P1 (Vm1, pH1) and P2 (Vm2, pH2)
Where P1 and P2 are points measured using the selected buffer solution. To determine the pH of an unknown solution, for a given point Px (Vmx, pHx), simple linear interpolation can be performed using equations:
(pHx – pH1) / (Vmx – Vm1)
= (pH1 – pH2) / (Vm1 – Vm2)
Or simply write as
pHx=
(Vmx – Vm1) × (pH1 – pH2)/(Vm1 – Vm2) + pH1
If there are multiple sets of points, to improve accuracy, use first-order linear regression. Given n data points P0 (Vm0, pH0), P1 (Vm1, pH1), P2 (Vm2, pH2), P3 (Vm3, pH3), ..., Pn (Vmn, pHn), the least squares method can be used to establish general Equation pHx = a + b ×Vmx, where b is the slope of the line and a is the intercept, the values ​​are as follows:
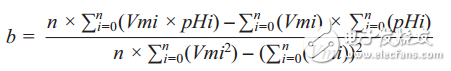
as well as

The least squares approximation method can be extended to higher order, such as second order nonlinear equations. The general second-order equation can be expressed as:
pHx = a + b × Vmx + c × Vmx2
The values ​​of a, b and c can be calculated as follows:
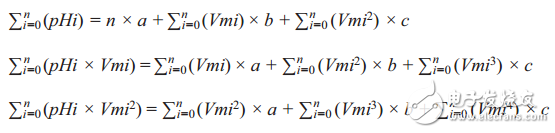
This system of equations can be solved by substitution, elimination or matrix methods to obtain the values ​​of the unknown variables a, b, c.
Part V: Hardware Design Solutions

Under this given condition, in order to isolate the circuit from the high source resistance, a buffer amplifier with high input impedance and ultra low input bias current is required. The AD8603 low noise op amp can be used as a buffer amplifier for this application. The low input current of the AD8603 minimizes the voltage error caused by the bias current flowing through the electrode resistor.
For a pH probe with a series resistance of 1 GΩ at 25 °C, the offset error is 0.2 mV (0.0037 pH) for a typical input bias current of 200 fA. Even at a maximum input bias current of 1 pA, the error is only 1 mV. Although not necessarily required, protection, shielding, high insulation resistance posts, and other such standard picoam methods can be utilized to minimize leakage at the high impedance input of the selected buffer.
Analog to digital converter
Low power ADCs are suitable for this application. The 16-bit ∑-Δ ADC AD7792 supports precision measurement applications. It has a low noise 3-channel input with a noise of only 40 nV rms at an update rate of 4.17 Hz. The device operates from a 2.7 V to 5.25 V supply and typically consumes 400 μA in a 16-pin TSSOP package. Other features include a built-in bandgap reference of 4 ppm/°C drift (typ), a shutdown power of up to 1 μA, and a built-in clock vibrator, resulting in reduced component count and PCB space.
Select RF transceiver
Based on the aforementioned requirements, ADuCRF101 is best suited for this application.
The ADuCRF101 is a fully integrated data acquisition solution for low-power wireless applications operating from 431 MHz to 464 MHz and 862 MHz to 928 MHz. It integrates communication peripherals such as the two SPI buses required for the application. On-chip 128 kB non-volatile Flash/EE memory and 16 kB SRAM are available. It is a single-chip solution that integrates microcontrollers and transceivers, which minimizes component count and board size.
The ADuCRF101 operates directly from a 2.2 V to 3.3 V battery range and consumes as follows: • 280 nA (shutdown mode, non-reserved state) • 1.9 μA (shutdown mode, processor memory and RF transceiver memory reserved) • 210 μA/MHz (Cortex-M3 processor in active mode) ? 12.8 mA (RF transceiver in receive mode, Cortex-M3 processor in shutdown mode) ? 9 mA to 32 mA (RF transceiver in transmit mode, Cortex- M3 processor is in shutdown mode)
Software Implementation
Software is one of the key parts of a wireless transmission system. It determines how the system works and also has an impact on system power consumption. The system has two software sections, the protocol stack and the application stack. The protocol stack used is ADRadioNet, a wireless network protocol for the ISM band. It uses IPv6 addresses and aggregates most of the features required for such solutions, such as low power, multi-hop, end-to-end replies, and self-healing. The application stack is software that accesses the pH reference design board via SPI.
In order to run the two software stacks efficiently, a simple scheduler is used. A non-preemptive scheduler handles protocol stack tasks, allocating a certain amount of time and resources to their functions. However, the number of tasks defined in the system is limited. In order to work efficiently, a non-preemptive scheduler must complete the execution of a defined task before its time elapses. For two stacks in the system, a non-preemptive scheduler is just right because the number of defined tasks assigned to it is limited.
Conclusion
This article describes the different challenges and solutions for pH wireless sensor monitoring design. ADI data acquisition products have proven to be used to address the challenges of pH measurement.
The AD8603 op amp or any equivalent ADI amplifier with high input impedance can be used to offset the high output impedance of the sensor, providing adequate shielding against system loading. The ADuCRF101 data acquisition system IC provides a complete RF data transmission solution. The high precision of data acquisition can be achieved by using either precision amplifiers and ADC hardware, or by software calibration, which uses mathematical statistics to create a general equation, such as various curve fitting methods.
Original text from Yadno Semiconductor
A TPU Screen Protector made of the super toughness of the honeycomb structure. Its unique ultra-soft properties allow it to cover the most complex curves and contours in a device.
The self-healing design of the Hydrogel Screen Protector can protect the display screen of the device from damage, leave no air bubbles, and maintain the sensitivity of the touch screen. Advanced anti-fingerprint and dust- and oleophobic overlays keep your screen smudge- and dirt-free. This overlay is also important in providing maximum touch sensitivity for improved high-speed glide and optimal touch response.
The optical transparency of the Hydrogel Film is more than 90%, showing you the most original screen color and bringing the most realistic visual experience.
If you want to know more about the product information of the Hydrogel Screen Protector for Xiaomi, please click the product details to view the parameters, model, picture, price and other information of the Xiaomi Screen Protector.
Whether you are a group or an individual, we will do our best to provide you with accurate and comprehensive information about Hydrogel Screen Protectors!
Srceen Protector For Xiaomi,Hydrogel Screen Protector For Xiaomi,TPU Screen Protector For Xiaomi,Hydrogel Film for Xiaomi
Shenzhen Jianjiantong Technology Co., Ltd. , https://www.jjthydrogelmachine.com