Abstract: Different optical colors (white light, A1; blue light, A2; red light, A3) and photoperiod (24L..0D, B1; 12L..12D, B2; 8L..16D, B3) were studied by orthogonal experiment. Effect of light intensity (0.88 W/m2, C1; 4.55 W/m2, C2; 8.60 W/m2, C3) on the growth and feeding of Atlantic salmon (Salmo salar) in aquaculture system (850.97±82.77) g . Experimental setup A1B1C1 (1), A1B2C2 (2), A1B3C3 (3), A2B1C2 (4), A2B2C3 (5), A2B3C1 (6), A3B1C3 (7), A3B2C1 (8), A3B3C2 (9) 9 treatment groups , raised for 180d under the corresponding setting conditions. The results show that the survival rate of Atlantic salmon is the highest when the light color is red, the photoperiod is 12L..12D and the light intensity is 8.60 W/m2, but the effects of light color, photoperiod and light intensity on survival rate are not significant ( P>0.05); The relative weight gain rate and fatness of the fish in each group were not significant (P>0.05). By the 120th day, the specific growth rate of the body length of the 2, 5, and 6 groups was significantly higher than that of the fish. Group 1 (P<0.05); By day 180, the specific growth rate of body mass in groups 1, 2, 4, 7, and 8 was significantly higher than that in group 6 (P<0.05), 1, 2, 3, 4, and 7 The daily weight gain of the groups 8 and 9 was significantly higher than that of the 6 groups (P<0.05). The body mass variation coefficient of the 9 groups was significantly lower than that of the 7 groups (P<0.05). The growth hormone in the plasma of the 9 groups was significantly higher than that in the 1, 2, 3, 4, 6, 7 and 8 groups (P<0.05). The illumination conditions were the best when the feeding rate, feed conversion efficiency and feed coefficient were: red light, 12L..12D, 8.60 W/m2, but the effects of light color, photoperiod and light intensity on feeding rate, feed conversion efficiency and feed coefficient were not significant (P>0.05). Under the experimental conditions, the suitable lighting conditions are: red light, 12L: 12D, 8.60 W/m2.
Salmo salar, commonly known as salmon, is native to the temperate and subarctic regions of the northern Atlantic Ocean and is one of the world's major farmed fish. It has a wide range of domestic and international markets. In recent years, it has been a good quality and good market prospect. The cultured species are introduced domestically. Atlantic salmon is a cold-water fish. The coastal waters in northern China have high water temperatures in summer and are not suitable for sea cage culture. The industrialized recirculating Aquaculture System (RAS) has the characteristics of water saving, environmental protection, high yield, no geographical and climatic restrictions, and can provide a stable and controllable environment for breeding animals. In the production process, the author found that the specific growth rate of Atlantic salmon in some recirculating aquaculture systems is low (0.33%~1.09%), and the feed coefficient is high (1.30~2.50), which seriously affects the development of the industry. It is necessary to study the growth and feeding patterns of Atlantic salmon under the model. Studies have shown that light environmental parameters are important factors affecting fish growth and feeding. Head et al. [1] showed that different light colors have a greater impact on the growth performance of the golden cockroach (Percaflavescens); Oppedal et al [2] reported the seasonal growth of the Atlantic salmon (Salmosalar) that the photoperiod migrated in the autumn. Harvest weight has a significant impact; Taylor et al. [3] have shown that both photoperiod and higher light intensity can be improved
The growth rate and feed conversion rate of rainbow trout (Oncorhynchus mykiss); Wang et al [4] found that the light intensity of 320~1 150 lx can significantly increase the survival rate and relative weight gain of juveniles of Epinephelus coioides. And specific growth rate. At present, the research on the combined effects of light color, photoperiod and light intensity on the growth performance and feeding of Atlantic salmon in recirculating aquaculture systems has not been reported. This study focuses on the effects of light, photoperiod and light intensity on the growth performance and feeding of Atlantic salmon under industrialized aquaculture mode, in order to obtain suitable light color for Atlantic salmon growth and feeding. Photoperiod and light intensity, and provide a theoretical reference for the application of LED lighting technology in the Atlantic 鲑 recirculating aquaculture system.
1 Materials and methods
1.1 Materials
The Atlantic salmon was provided by the Fish Research Center of Shandong Oriental Ocean Technology Co., Ltd. During the experiment, the healthy fish with body length (39.71±1.58) cm and body mass (850.97±82.77) g were selected. The experiment uses Norway's Skretting squid pellet feed. The main nutrients are: crude protein ≥ 48.0%, crude fat ≥ 18.0%, crude fiber ≤ 1.0%, and coarse ash ≤ 12.0%. Experimental system: 3 sets of recirculating aquaculture experimental system designed by ourselves, 3 culture tanks per system, composed of culture tank, solid-liquid separator, physical filtration, foam separator, ultraviolet sterilization device, biological filter and other units (figure 1). The culture pond is a round glass-steel structure with a diameter of 200 cm, a pool height of 130 cm and an effective water depth of 105 cm. The volume of water in each culture pond is 3 297 L. The influent flows along the side wall of the tank wall. The liquid flow meter measures and controls the water inflow, and drains through the drain pipe in the center of the tank. The experiment used a white (l550 nm), blue (l455 nm) and red (l625 nm) COB integrated package LED light source. The lamps were designed by the Institute of Semiconductors of the Chinese Academy of Sciences and manufactured by Jiangsu Wuxi Huazhao Optoelectronics Technology Co., Ltd. The settings for light intensity and photoperiod levels are shown in Table 1. The light intensity is determined by the measured value of 5 cm above the center of the water surface, and the height of the floodlight is adjusted to ensure the light intensity of each experimental group. During the experiment, the shade cloth was placed between adjacent culture ponds to prevent mutual contamination of light between different light sources. The light color and light intensity were measured by SP-10 spectrometer developed by Hangzhou Yuanfang Optoelectronic Information Co., Ltd., and the photoperiod was adjusted by time controller.
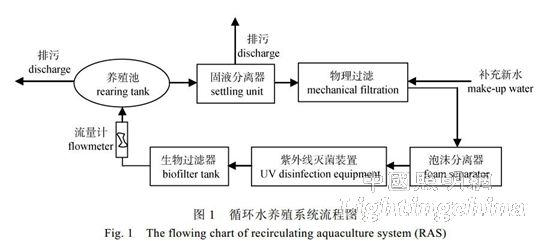
1.2 Methods
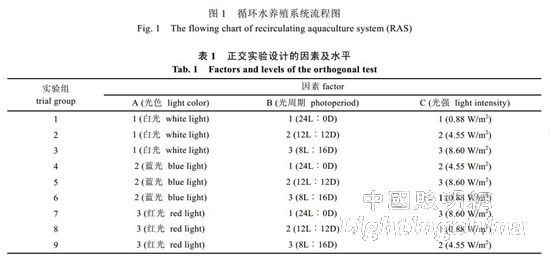
1.2.1 Experimental design and management experiments were conducted from August 2012 to March 2013 for a period of 180 days. The experiment uses L9 (33) orthogonal design, setting three factors of light color, photoperiod and light intensity, each factor is set at 3 levels (Table 1), a total of 9 processing combinations: A1B1C1, A1B2C2, A1B3C3, A2B1C2, A2B2C3 , A2B3C1, A3B1C3, A3B2C1, A3B3C2. 60 fish were cultured in each treatment combination (30 tails with PER perforation mark, 30 tails not marked), for a total of 540 tails. During the experiment, the compound feed was fed twice at 7:30 and 14:00 every day. The amount of feed was based on full food. Each time the bait is taken, the feeding of the fish is stopped when the feeding is not active. After each feeding for 40 min, the gauze bag was used to collect the residual bait and feces at the drain of the solid-liquid separator, the amount of unfed bait was recorded, and the actual food intake was calculated. In the experimental stage, the water temperature was maintained at (15.7±0.4) °C, salinity was 24~26, pH was 7.2~7.5, dissolved oxygen saturation was 100%~140%, and total ammonia nitrogen (TAN) was <0.25 mg/L. The new water quantity is adjusted according to the measured water quality data, and the general water exchange amount does not exceed 10%. 1.2.2 Sample collection After the start of the experiment, the fish were starved for 1 d per month, and the mass and body length, fork length, and the amount of residual feed, and the amount of residual bait, measured and unlabeled in each treatment group, were measured. Growth and feeding performance. Each group of labeled Atlantic salmon blood was collected on an empty stomach every month to prepare plasma and to measure growth hormone. At the end of feeding, the survival rate of each treatment group was counted.
1.2.3 Parameter calculation formula
Survival rate (SR): SR (%) = 100 × Nf / Ni [5]
Specific growth rate (SGR): SGR (%/d) = 100 × (lnBW2 - lnBW1) / (T2 - T1) [6] Body length specific growth rate (SGRBL): SGRBL (%/d )=100×(lnBL2–lnBL1)/(T2–T1)[6] Daily weight gain (DWG): DWG (g/d)=(BW2–BW1)/(T2–T1)[6] Relative weight gain (RWG): RWG (%)=100×BW2/BW1[7] Condition factor (CF): CF=BW/FL3[7]
Coefficient of size variation (SV): SV=100×SD/X[6]
Food intake (FI): FI (%/d) = 100 × F / [0.5 × (BW2 + BW1) × (T2 - T1)] [6]
Food conversion efficiency (FCE): FCE (%) = 100 × (BW2 – BW1) / F [5]
Feed conversion ratio (FCR): FCR=F/(BW2–BW1)[6] where Ni and Nf are the initial number of fishtails and the final number of fishtails; BW1 and BW2 are the initial body masses of each fish. And final body mass (g); T1, T2 are the time corresponding to BW1 and BW2, respectively; BL1 and BL2 are the initial body length and final body length (cm) of fish respectively; BW is the body mass (g) of each fish; FL is the length of each harpoon (cm); F is the total food intake (g); SD is the standard deviation; X is the average body mass (g) of the treated group of fish.
1.2.4 Determination of growth hormone After PER perforated labeled fish were weighed, blood was taken from the living tail vein. The blood sample was first injected into a centrifuge tube containing heparin sodium anticoagulated in crushed ice, and then centrifuged at 5 000 r/min. At 10 min, plasma was stored at –70 °C and the growth hormone (GH) content was measured. The concentration of growth hormone (GH) in plasma was measured by radioimmunoassay established by Björnsson et al. [8]. The GH radioimmunoassay kit was provided by Beijing North Biotechnology Research Institute.
1.3 Statistical analysis
The experimental data were statistically analyzed using SPSS18.0 software, and one-way ANOVA was used to compare the significance between the data with the least significant difference method (LSD). Data were expressed as mean ± standard deviation (x ± SD), and significant differences were observed at P < 0.05.
2 Results and analysis
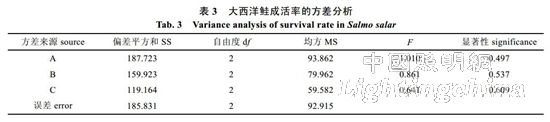
2.1 Effects of light color, photoperiod and light intensity on the survival rate of Atlantic salmon The survival rates of Atlantic salmon in treatment groups 6 and 9 were significantly lower than those in other groups (P<0.05, Table 2), indicating 8L..16D and 0.88W. The combined condition of /m2 or 4.55 W/m2 is not conducive to the survival of Atlantic salmon in the circulating water system. The results of 9 groups of experiments were classified and analyzed, and the light color conditions for the formation of Atlantic salmon from high to low were: white light (95.00%), red light (90.55%), blue light (83.89%); photoperiod conditions were: 12L ..12D (93.33%), 24L..0D (92.22%), 8L..16D (83.89%); The light intensity is: 8.60 W/m2 (94.44%), 0.88 W/m2 (89.44%), 4.55 W/m2 (85.55%). Therefore, Atlantic salmon has the highest survival rate under conditions of white light, 12L..12D, and 8.60 W/m2. There is no significant difference between the white light and the red light of the A factor, so the A factor selects red light, and finally the red light, 12L..12D, and 8.60 W/m2 are the best conditions for the culture of Atlantic salmon. The analysis of variance showed that (Table 3), light color, photoperiod and light intensity had no significant difference in the survival rate of Atlantic salmon (P>0.05). Therefore, it is believed that during the growth of Atlantic salmon, light color, photoperiod and light intensity have not had a significant impact on the survival rate of Atlantic salmon.
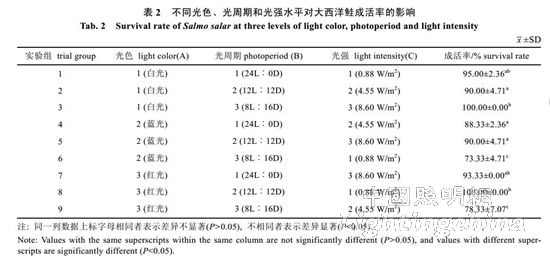
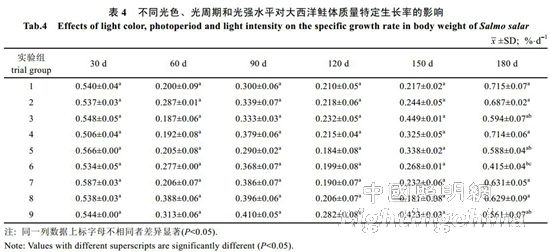
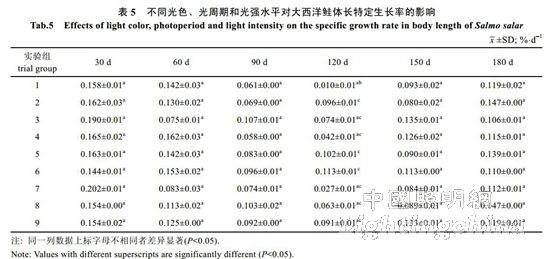
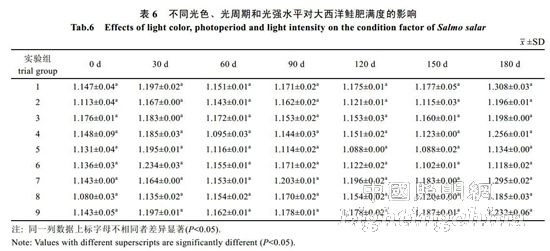
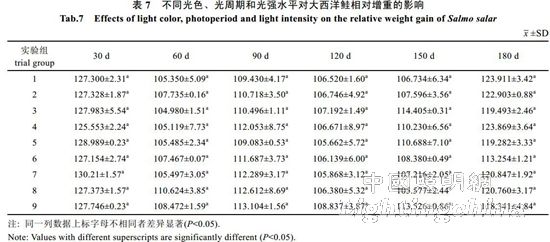
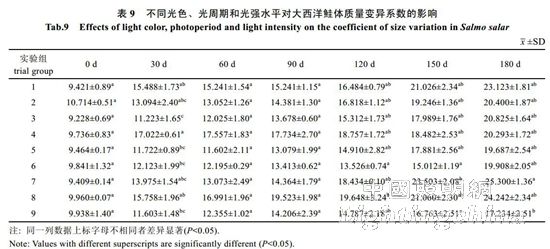
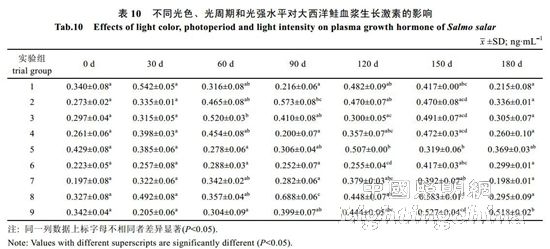
2.2 The effect of light color, photoperiod and light intensity on the growth of Atlantic salmon is shown in Table 4. The specific growth rate of Atlantic carcass mass varies from 0.18 to 0.71%/d, showing an irregular "W" trend. On the 30th, 60th, 90th, 120th and 150th day of the experiment, there was no significant difference in the specific growth rate of each group (P>0.05). By the 180th day, the difference between groups 1, 2, 4, 7 and 8 was not significant (P> >0.05), but both were significantly higher than 6 groups (P<0.05). The specific growth rate of each group was from high to low: 1 group, 4 groups, 2 groups, 7 groups, 8 groups, 3 groups, 5 Group, 9 groups, 6 groups. It can be seen from Table 5 that the specific growth rate of the body length varies between 0.01 and 0.20%/d. On the 30th, 60th, 90th, 150th and 180th days of the experiment, the specific growth rate of each body length is not significantly different (P>0.05). On the 120th day, groups 2, 5, and 6 were significantly higher than group 1 (P < 0.05). From Table 6 and Table 7, there was no significant difference in the fullness and relative weight gain of the fish in the whole experimental period (P>0.05), and the range of variation was between 1.08~1.31 and 104.98%~130.00%, respectively. From Table 8, it is concluded that the daily weight gain varies from 2.07 to 11.91 g/d, and the daily weight gain characteristics of each group of fish are similar to the specific growth rate of body mass, to the 30th, 60th, 90th, 120th, and 150th days. There was no significant difference in daily weight gain between the groups (P>0.05). On the 180th day, groups 1, 2, 3, 4, 7, 8 and 9 were significantly higher than group 6 (P<0.05). From high to low, they are: 4 groups, 1 group, 2 groups, 7 groups, 8 groups, 9 groups, 3 groups, 5 groups, 6 groups. From Table 9, it can be seen that the difference in body mass variation coefficient of each group before the experiment was not significant (P>0.05); on the 30th day after the experiment, the three groups were significantly lower than the 1, 4, and 8 groups (P<0.05), while 5 and 6 and The 9 groups were significantly lower than the 4 groups (P<0.05); the differences were not significant in the 60th and 90th days (P>0.05); the 6 groups were significantly lower than the 8 groups (P<0.05) by the 120th day; Groups 6 and 9 were significantly lower than 7 groups (P<0.05); to 180 days, 9 groups were significantly lower than 7 groups (P<0.05). The coefficient of body mass variation from 9 to 5 in each group was from low to high. 6 groups, 4 groups, 2 groups, 3 groups, 1 group, 8 groups, 7 groups. The levels of growth hormone in plasma of Atlantic salmon under different light conditions varied with time (Table 10). At the beginning of the experiment and on the 30th day of the experiment, there was no significant difference in the concentration of growth hormone in the plasma of each group (P>0.05). By the 60th day of the experiment, the 3 groups were significantly higher than the 5, 6 and 9 groups (P<0.05). By the 90th day, the 8 groups were significantly higher than the 1, 3, 4, 5, 6, 7, and 9 groups (P < 0.05), while the 2 groups were significantly higher than the 1, 4, 6 and 7 groups (P < 0.05). ); By day 120, groups 1, 2, 5, and 8 were significantly higher than group 6 (P < 0.05), and groups 5 were significantly higher than group 3 (P < 0.05); to day 150, 2, 3, 4 and Group 9 was significantly higher than group 5 (P<0.05), group 8 was significantly higher than group 1,5,6 and 7 (P<0.05), 9 group was significantly higher than group 7 (P<0.05); to 180 days, Group 9 was significantly higher than groups 1, 2, 3, 4, 6, 7, and 8 (P < 0.05), with 9 groups having the highest growth hormone content (0.52 ng/mL) (Table 10).
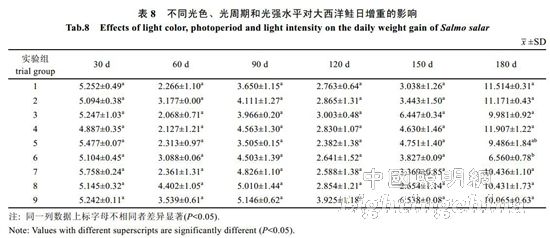
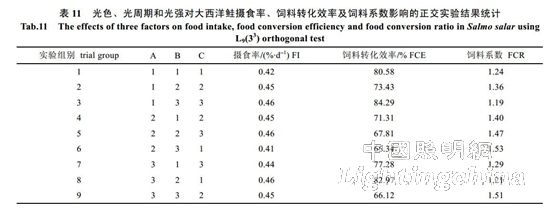
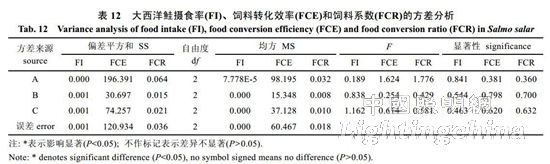
2.3 Effects of light color, photoperiod and light intensity on the feeding of Atlantic salmon The experimental results show that the light intensity has the greatest influence on the feeding rate (FI) of Atlantic salmon, followed by photoperiod and light color. The best combination is C3B2A3, ie 8.60 W/ M2, 12L..12D, red light (Table 11). Light color has the greatest influence on feed conversion efficiency (FCE), followed by light intensity and photoperiod. The best combination is A1C3B1, namely white light, 8.60W/m2, 24L..0D; A1 and A3, B1 and B2 in A and B factors. There is no significant difference between the levels, so the A and B factors are taken as A3 and B2 respectively. The best condition determined is A3C3B2, namely red light, 8.60 W/m2, 12L..12D. Light color has the greatest influence on feed coefficient (FCR), followed by photoperiod and light intensity. The best level combination is A2B3C2, ie blue light, 8L..16D, 4.55 W/m2. The final conditions determined were red light, 12L..12D, 8.60 W/m2. The analysis of variance and F test of the data in Table 11 showed (Table 12) that under the experimental conditions, light color, photoperiod and light intensity had no significant effect on the feeding rate, feed conversion efficiency and feed coefficient of Atlantic salmon (P>0.05). ).
3 Discussion
3.1 Effects of light color, photoperiod and light intensity on the survival rate of Atlantic salmon Light color, photoperiod and light intensity are important environmental factors affecting the survival rate of teleost fish. Downing [9] reported the effect of spectral composition on the survival rate of Melanogrammus aeglefinus. Trotter et al. [10] found that a longer photoperiod can increase the survival rate of Latris lineata, and 24L..0D has a higher survival rate than the photoperiod containing dark phase. Puvanendran et al. [11] showed that the survival rate of the Atlantic cockroach (Gadusmorhua) was significantly higher than that of the 300, 600, and 1200 lx groups at 2 400 lx light intensity. The experimental results show that under the conditions of red light, 12L..12D and 8.60 W/m2, the survival rate of Atlantic salmon is the highest. Wang Jiqiao et al [12] showed that æˆ (Hypophthalmichthys molitrix) and é³™ (Aristichthys nobilis) had the highest survival rate at 10-12 h after sunshine. The study by Yoseda et al. [13] found that the survival rate of Plectropomus leopardus was significantly higher than that of low light intensity (0 and 500 lx) at high light intensity of 3 000 and 1000 lx. These observations are consistent with the results of this experiment, indicating that under red light conditions, the photoperiod of 12L..12D and higher light intensity (8.60 W/m2) can increase the survival rate of Atlantic salmon.
3.2 The effects of light color, photoperiod and light intensity on the growth of Atlantic salmon have been reported. Photoperiod, light intensity and spectral composition (light color) are important factors affecting the physiological response, growth and behavior of teleost fish [14–16] . In this experiment, body mass, body length specific growth rate, body mass difference coefficient, daily weight gain and plasma growth hormone were selected as indicators to reflect the growth of Atlantic salmon. Among the salmonids, the main role of growth hormone is associated with growth, metabolism, and osmotic balance regulation [17]. The results of this experiment show that, with the passage of time, the effects of light color, photoperiod and light intensity on the specific growth rate, specific growth rate, body mass difference coefficient and daily weight gain of Atlantic carcass are different. Plasma levels of growth hormone were significantly higher in treatment group 9 (red light, 8 L..16D, 4.55 W/m2) on days 180 than in groups 1, 2, 3, 4, 6, 7, and 8. It has been reported that Karakatsouli et al [18] studied the cockroach (Cyprinuscarpio) and showed that compared with white light and blue light, red light can significantly promote the specific growth rate and body mass increase of body mass. Studies by Endal et al. [19] have shown that long photoperiod or continuous illumination can improve the growth performance of the Atlantic salmon in the spring. Stefansson et al. [20] found that continuous illumination promotes the growth of Atlantic salmon compared to the natural photoperiod, and the growth rate increases with increasing light intensity. A study by Oppedal et al. [21] showed that high light intensity significantly promoted the growth of Atlantic salmon in cages. Studies have also pointed out that the effect of photoperiod on growth may be caused by the "light-pituitary axis" stimulating the pituitary to increase the synthesis and secretion of growth hormone [22].
3.3 Effects of light color, photoperiod and light intensity on Atlantic salmon feeding Light color, photoperiod and light intensity are important conditions for fish feeding. Studies by Luchiari et al. [23] have shown that long wavelengths can increase the feeding rate and feed efficiency of the scorpionfish (Sander lucioperca). Biswas et al. [24] showed that the feeding rate and feed conversion efficiency under photoperiod 24L..0D were significantly higher than 16L..8D, 6L..6D, 12L..12D. Studies by Strand et al. [25] have shown that light intensity can significantly affect the feeding rate of Perca fluviatilis. In this experiment, the effects of light environmental factors on the feeding rate, feed conversion efficiency and feed coefficient of Atlantic salmon are as follows: light intensity, photoperiod, light color, light color, light intensity, photoperiod and light color, The optimal combination of photoperiod and light intensity is 8.60 W/m2, 12L..12D, and red light. The results of variance analysis showed that light color, photoperiod and light intensity did not significantly affect the feeding rate, feed conversion efficiency and feed coefficient of Atlantic salmon. There are some differences between the results of this experiment and the reports of previous literatures. The reasons for the differences are further studied.
4 Conclusion
In industrialized aquaculture systems, light color, photoperiod and light intensity have little effect on the growth and feeding of Atlantic salmon. Red light, 12L..12D and 8.60 W/m2 are suitable light conditions under the experimental conditions. As a new energy-saving light source, LED lamp has a good application prospect for regulating the growth and feeding of fish in recirculating aquaculture systems.
references:
[1] Head AB, Malison J A. Effects of lighting spectrum anddisturbance level on the growth and stress responses of yellow perch Perca flavescens [J]. J World Aquacult Soc, 2000, 31 (1): 73–80.
[2] Oppedal F, Berg A, Olsen RE, et al. Photoperiod in seawaterinfluence seasonal growth and chemical composition in autumn sea transferred Atlantic salmon (Salmo salar L.) giventwo vaccines[J]. Aquaculture, 2006, 254 (1–4 ): 396– 410.
[3] Taylor JF, North BP, Porter MJR, et al. Photoperiod canbe used to enhance growth and improve feeding efficiency infarmed rainbow trout, Oncorhynchus mykiss [J]. Aquaculture, 2006, 256 (1–4): 216–234.
[4] Wang T, Cheng YZ, Liu ZP, et al. Effects of light intensityon growth, immune response, plasma cortisol and fatty acid composition of juvenile Epinephelus coioides reared in artificial seawater [J]. Aquaculture, 2013, 414: 135–139 .
[5] Hu Bin, Li Xiaoqin, Leng Xiangjun, et al. Effects of feed Vc on grass carp growth, muscle quality and non-specific immunity[J]. Chinese Journal of Fisheries Sciences, 2008, 15 (5): 794–800.
[6] Zhang Jianming, Guo Baifu, Gao Yong. Growth, feeding and behavioral responses of juvenile Chinese sturgeon to chronic crowding stress[J]. Chinese Journal of Fisheries Sciences, 2013, 20(3): 592–598.
[7] Leclercq E, Taylor JF, Sprague M, et al. The potential of alternative therapy-systems to suppress pre-harvest sexualmaturation of 1+ Atlantic salmon (Salmo salar) post-smoltsreared in commercial sea-cages [J]. Aquaculture, 2011, 44 (2): 35–47.
[8] Björnsson BT, Taranger GL, Hansen T, et al. The interrelation between photoperiod, growth hormone and sexual maturation of adult Atlantic salmon (Salmo salar) [J]. GenComp Endocrinol, 1994, 93 (1): 70–81.
[9] Downing G. Impact of spectral composition on larval haddock, Melanogrammus aeglefinus L., growth and survival[J].Aquacult Res, 2002, 33 (4): 251–259.
[10] Trotter AJ, Battaglene SC, Pankhurst P M. Effects of photoperiod and light intensity on initial swim bladderinflation, growth and post-inflation viability in culturedstriped trumpeter (Latris lineata) larvae[J]. Aquaculture,2003, 224 (1–4 ): 141–158.
[11] Puvanendran V, Brown J A. Foraging, growth and survival of Atlantic cod, Gadus morhua, larvae reared in differentlight intensities and photoperiods [J]. Aquaculture, 2002, 214 (1–4): 131–151.
[12] Wang Jiqiao, Zhao Deshu, Zhang Jingquan. Effects of different sunshine hours on the growth and survival rate of carp, carp and carp fry [J]. Chinese Journal of Ecology, 1994, 13(3): 41–44.
[13] Yoseda K, Yamamoto K, Asami K, et al. Influence of lightintensity on feeding, growth, and early survival of leopardcoral grouper (Plectropomus leopardus) larvae undermass-scale rearing conditions[J]. Aquaculture, 2008, 279(1) –4): 55–62.
[14] Oppedal F, Taranger GL, Juell JE, et al. Growth, osmoregulation and sexual maturation of underyearling Atlanticsalmon smolt Salmo salar L. exposed to different intensities of continuous light in sea cages [J]. Aquacult Res, 1999, 30(7) ): 491–499.
[15] Boeuf G, Le Bail P Y. Does light have an influence on fishgrowth? [J]. Aquaculture, 1999, 177 (1–4): 129–152.
[16] Karakatsouli N, Papoutsoglou SE, Pizzonia G, et al. Effects of light spectrum on growth and physiological status of gilthead seabream Sparus aurata and rainbow trout Oncorhynchus mykiss reared under recirculating system conditions[J].Aquacult Eng, 2007, 36 (3 ): 302–309.
[17] Björnsson B T. The biology of salmon growth hormone: from daylight to dominance [J]. Fish Physiol Biochem, 1997,17 (1/6): 9–24.
[18] Karakatsouli N, Papoutsoglou SE, Sotiropolos N, et al. Effects of light spectrum, rearing density and light intensity ongrowth performance of scaled and mirror common carp Cyprinus carpio reared under recirculating system conditions[J].Aquacult Eng, 2010, 42 (3): 121–127.
[19] Endal HP, Taranger GL, Stefansson SO, et al. Effects of continuous additional light on growth and sexual maturity inAtlantic salmon, Salmo salar, reared in sea cages [J]. Aquaculture, 2000, 191 (4): 337–349 .
[20] Stefansson SO, Hansen T, Taranger G L. Growth and parr-smolt transformation of Atlantic salmon (Salmo salar) under different light intensities and subsequent survival and growth in seawater [J]. Aquacult Eng, 1993, 12 (4): 231 –243.
[21] Oppedal F, Taranger GL, Juell J, et al. Light intensity affects growth and sexual maturation of Atlantic salmon (Salmo salar L.) postsmolts in sea cages [J]. Aquat LivingRes, 1997, 10: 351–357.
[22] Björnsson BT, Thorarensen H, Hirano T, et al. Photoperiod and temperature affect plasma growth hormone levels, growth, condition factor and hypoosmoregulatory ability of juvenile Atlantic salmon (Salmo salar) during parr-smolttransformation [J]. Aquacultue, 1989, 82 (1–4): 77–91.
[23] Luchiari AC, Freire FAM, Pirhonen J, et al. Longer wavelengths of light improve the growth, intake and feed efficiency of individual reared juvenile pike perch Sanderlucioperca (L.) [J]. Aquacult Res, 2009, 40 (8) ): 880–886.
[24] Biswas AK, Seoka M, Inoue Y, et al. Photoperiod influences the growth, food intake, feed efficiency and digestibility of red sea bream (Pagrus major) [J]. Aquaculture, 2005, 250 (3–4): 666– 673.
[25] Strand Å, Alanärä A, Staffan F, et al. Effects of tank colour and light intensity on feed intake, growth rate and energyexpenditure of juvenile Eurasian perch, Perca fluviatilis L. [J]. Aquaculture, 2007, 272 (1– 4): 312–318.Effects of different types of environment light on the growth performance and feeding of Atlantic salmon (Salmo salar) in recirculating aquaculture systemsQIU Denggao1, 2, 3, XU Shihong1, LIU Ying1, SONG Changbin4, CHI Liang1, WANG Shunkui5 , YU Kaisong5
1. Institute of Oceanology, Chinese Academy of Sciences, Qingdao 266071, China;
2. University of Chinese Academy of Sciences, Beijing 100049, China;
3. Fisheries Research Institute of Fujian, Xiamen 361012, China;
4. Institute of Semiconductors, Chinese Academy of Sciences, Beijing 100083, China;
5. Shandong Oriental Ocean Sci-Tech Co. Ltd., Yantai 264003, China
Abstract: We aim to explore the effects of different types of light color (ie, A1, white light; A2, blue light; A3, red light), photoperiod (ie, B1, 24L..0D; B2, 12L..12D; B3 , 8L..16D) and light intensity (ie, C1, 0.88 W/m2; C2, 4.55 W/m2; C3, 8.60 W/m2) on the growth performance and feeding of Atlantic salmon (Salmo salar) with initial body weights of 850.97±82.77 g for 180 days in recirculating aquaculture systems (RAS) using orthogonal test methods. Nine treatment groups were designed in the present experiment: A1B1C1(group1), A1B2C2(group2), A1B3C3(group3), A3B3C2(Group4), A2B2C3 (group5), A2B3C1(group6), A3B1C3(group7), A3B2C1(group8), and A1B1C1(group9). The results showed that the highest survival rate was found in red light, 12L..12D and 8.60 W/m2 at the end Of the trial, andno significant difference was observed in the survival rates of the experimental groups (P>0.05). There were no significant differences in relative weight gain and condition among the nine groups at each At day120, the specific growth rate of body length for groups 2, 5 and 6 were significantly higher than those of group 1 (P<0.05). At day 180, the specific growth rate of body weight In groups 1, 2, 4, 7 and 8 were significantly higher thanthose of group 6 (P<0.05), and the daily weight gain of groups 1, 2, 3, 4, 7, 8 and 9 showed higher variation than thoseof group 6 (P<0.05); the coefficient of size variation (SV) in group 9 was lower than in group 7 (P<0.05). Additionally, the plasma growth hormone in group 9 was a higher concentration than that of groups 1, 2 , 3, 4, 6, 7 and 8 (P<0.05). Better food intake (FI), food conversion efficiency (FCE) and food conversion ratio (FCR) were also observed in red light (12L..12D) at 8.60 W /m2 at the end of the experiment, while light color, photoperiod and light intensity had no significant effects on the FI, FCE and FCR between groups (P>0.05). Hence, red light (12L..12D) at 8.60 W/m2 Is considered to be a suitable combinati On of artificial lighting under the experimental conditions described herein.
Key words: Salmo salar; light color; photoperiod; light intensity; growth performance; feeding; recirculating aquaculture systems
Effects of photoenvironment factors on the growth and feeding of Atlantic salmon in recirculating aquaculture systems
references:
1.Head AB, Malison J A. Effects of lighting spectrum and disturbance level on the growth and stress responses of yellow perch Perca flavescens [J]. J World Aquacult Soc, 2000, 31(1): 73-80. 2000
2.Oppedal F, Berg A, Olsen RE, et al. Photoperiod in seawater influence seasonal growth and chemical composition in au-tumn sea transferred Atlantic salmon (Salmo salar L.)given two vaccines[J]. Aquaculture, 2006, 254( 1-4): 396-410.2006
3.Taylor JF, North BP, Porter MJR, et al. Photoperiod can be used to enhance growth and improve feeding efficiency in farmed rainbow trout, Oncorhynchus mykiss[J]. Aquaculture, 2006, 256(1-4):216-234 . 2006
4.Wang T, Cheng YZ, Liu ZP, et al. Effects of light intensity on growth, immune response, plasma cortisol and fatty acid composition of juvenile Epinephelus coioides reared in arti-ficial seawater[J]. Aquaculture, 2013, 414: 135-139. 2013
5. Hu Bin, Li Xiaoqin, Leng Xiangjun, Li Jiale, Effects of Wenhua Feed VC on grass carp growth, muscle quality and non-specific immunity [期刊论文]-China Fisheries Science 2008(05)
6. Zhang Jianming, Guo Baifu, Gao Yong, Chinese sturgeon, growth, feeding and behavioral responses to chronic crowding stress [期刊论文]-China Fisheries Science 2013(03)
7. Leclercq E, Taylor JF, Sprague M, et al. The potential of alternative lighting-systems to suppress pre-harvest sexual maturation of 1+ Atlantic salmon (Salmo salar) post-smolts reared in commercial sea-cages [J]. Aquaculture, 2011, 44(2): 35-47. 2011
8. Björnsson BT, Taranger GL, Hansen T, et al. The interre-lation between photoperiod, growth hormone and sexual maturation of adult Atlantic salmon (Salmo salar) [J]. Gen Comp Endocrinol, 1994, 93(1): 70 -81. 1994
9.Downing G. Impact of spectral composition on larval had-dock, Melanogrammus aeglefinus L., growth and survival[J]. Aquacult Res, 2002, 33(4):251-259. 2002
10.Trotter AJ, Battaglene SC, Pankhurst P M. Effects of photoperiod and light intensity on initial swim bladder inflation, growth and post-inflation viability in cultured striped trumpeter (Latris lineata) larvae [J]. Aquaculture, 2003, 224 (1) -4): 141-158. 2003
11. Puvanendran V, Brown J A. Foraging, growth and survival of Atlantic cod, Gadus morhua, larvae reared in different light intensities and photoperiods [J]. Aquaculture, 2002, 214(1-4): 131-151. 2002
12.Wang Jiqiao, Zhao Deshu, Zhang Jingquan. Effects of different sunshine hours on the growth and survival rate of carp, carp and carp fry [J]. Chinese Journal of Ecology, 1994, 13(3): 41-44.
13.Yoseda K, Yamamoto K, Asami K, et al. Influence of light intensity on feeding, growth, and early survival of leopard coral grouper (Plectropomus leopardus) larvae under mass-scale rearing conditions [J]. Aquaculture, 2008, 279 (1-4): 55-62.2008
14.Oppedal F, Taranger GL, Juell JE, et al. Growth, osmore-gulation and sexual maturation of underyearling Atlantic salmon smolt Salmo salar L. exposed to different intensities of continuous light in sea cages [J]. Aquacult Res, 1999, 30(7): 491-499.
15.Boeuf G, Le Bail P Y. Does light have an influence on fish growth [J]. Aquaculture, 1999, 177(1-4):129-152. 1999 16.Karakatsouli N, Papoutsoglou SE, Pizzonia G, et Al. Effects of light spectrum on growth and physiological status of gilt-head seabream Sparus aurata and rainbow trout Oncorhy-nchus mykiss reared under recirculating system conditions[J]. Aquacult Eng, 2007, 36(3): 302-309. 2007
17.Björnsson B T. The biology of salmon growth hormone: from daylight to dominance [J]. Fish Physiol Biochem, 1997, 17(1/6): 9-24. 1997
18.Karakatsouli N, Papoutsoglou SE, Sotiropolos N, et al. Ef-fects of light spectrum, rearing density and light intensity on growth performance of scaled and mirror common carp Cy-prinus carpio reared under recirculating system conditions[J]. Aquacult Eng , 2010, 42(3): 121-127. 2010
19. Endal HP, Taranger GL, Stefansson SO, et al. Effects of continuous additional light on growth and sexual maturity in Atlantic salmon, Salmo salar, reared in sea cages [J]. Aqua-culture, 2000, 191(4): 337-349. 2000
20. Stefansson SO, Hansen T, Taranger G L. Growth and parr-smolt transformation of Atlantic salmon (Salmo salar) under different light intensities and subsequent survival and growth in seawater [J]. Aquacult Eng, 1993, 12(4): 231-243. 1993
21. Oppedal F, Taranger GL, Juell J, et al. Light intensity af-fects growth and sexual maturation of Atlantic salmon (Salmo salar L.) postsmolts in sea cages [J]. Aquat Living Res, 1997, 10:351- 357. 1997
22. Björnsson BT, Thorarensen H, Hirano T, et al. Photoperiod and temperature affect plasma growth hormone levels, growth, condition factor and hypoosmoregulatory ability of juvenile Atlantic salmon (Salmo salar) performing parr-smolt transformation[J]. Aquacultue, 1989 , 82(1-4): 77-91. 1989
23. Luchiari AC, Freire FAM, Pirhonen J, et al. Longer wave-lengths of light improve the growth, intake and feed effi-ciency of visible reared juvenile pike perch Sander lucioperca (L.) [J]. Aquacult Res, 2009 , 40(8): 880-886.2009
24. Biswas AK, Seoka M, Inoue Y, et al. Photoperiod influences the growth, food intake, feed efficiency and digestibility of red sea bream (Pagrus major) [J]. Aquaculture, 2005, 250(3-4): 666 -673. 2005
25.Strand Å, Alanärä A, Staffan F, et al. Effects of tank colour and light intensity on feed intake, growth rate and energy expenditure of juvenile Eurasian perch, Perca fluviatilis L. [J]. Aquaculture, 2007, 272(1 -4): 312-318. 2007
Gold Finger Pcb,Flexible Circuit Board Gold Finger,Circuit Board Gold Finger Plating,Gold Finger Circuit Board
Shenzheng Weifu Circuit Technology Co.Ld , https://www.wfcircuit.com